Alain Pralong1,3 Renaud Jacquemart2,3 Katrina Cordovado2,3
- Pharma Consulting ENABLE GmbH
- Omnium Global Consulting Inc.
- ENABLE Biotech AG
Introduction
Exosomes have rapidly evolved from a scientific curiosity into one of the most promising platforms in next-generation therapeutics. These naturally secreted extracellular vesicles carry proteins, lipids, and nucleic acids capable of modulating cellular behavior, influencing immune responses, and promoting regeneration¹. Their potential as delivery vehicles and therapeutic agents is substantial—but so are the manufacturing challenges required to translate that potential into clinical reality.
Like other complex biologics, exosomes are profoundly shaped by their source cells, culture conditions, and isolation methods. These dependencies make consistency, scalability, and regulatory alignment the critical barriers standing between today’s scientific promise and tomorrow’s approved products. For innovators advancing exosome therapies, partnering with a CDMO that understands these nuances is essential.
Current Bottlenecks in Exosome Production
Despite two decades of research, exosome manufacturing still faces foundational obstacles. Variability in cell culture conditions, donor-dependent biology, and sensitivity to shear forces complicate upstream processes¹. Downstream, exosomes aggregate easily, are cleared rapidly in vivo, and often require surface modifications to extend circulation or enhance targeting.
Isolation methods further influence reproducibility. Many programs still rely on legacy ultracentrifugation workflows that are labor-intensive, low throughput, and difficult to standardize³ at scale. Removing contaminants—such as proteins, nucleic acids, lipoproteins, and cell debris—without damaging vesicles remains a persistent challenge. Compounding these issues is the lack of universally accepted potency assays or consistent dose definitions across particle number, protein mass, or biological activity.
Together, these factors create significant CMC risk and make it difficult for developers to demonstrate that each manufacturing batch is comparable, functional, and safe.
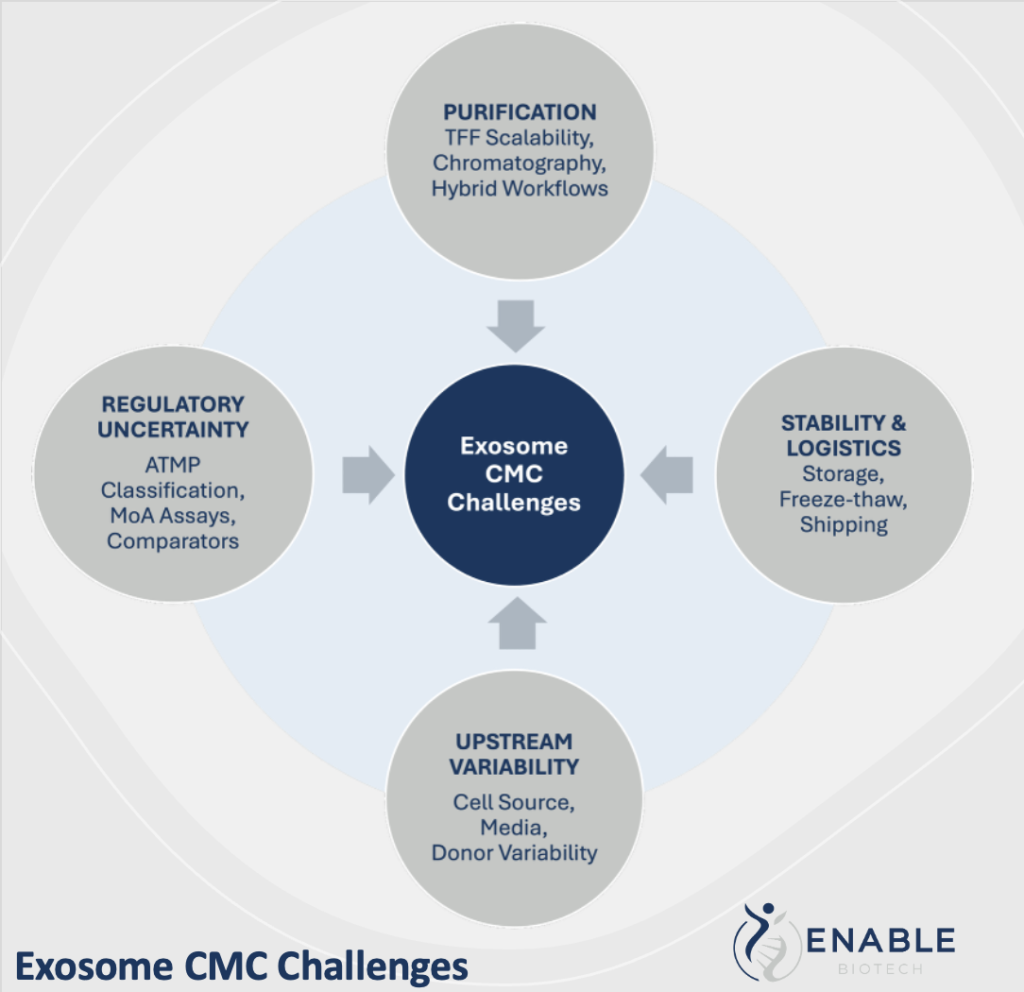
Advances in Purification Technologies
Scalable, GMP-compatible purification is a defining success factor for exosome therapies. Over the past several years, several technologies have emerged as more practical and reproducible alternatives to ultracentrifugation.
Tangential flow filtration (TFF) has rapidly become the backbone of modern EV purification. Optimized membranes and reduced shear stress⁴-⁵ make TFF gentler and far more scalable, and its compatibility with closed systems aligns well with GMP expectations.
Chromatography has also gained traction:
- Size-exclusion chromatography (SEC): simple, cost-effective, and suitable for polishing⁶ steps.
- Immunoaffinity chromatography: highly selective, ideal for subtype enrichment⁷ or engineered exosomes.
Emerging technologies—membrane affinity capture, microfluidic separation, and nanoporous magnetic platforms—offer intriguing selectivity but vary in maturity and scalability³. Increasingly, developers are adopting hybrid purification workflows that combine complementary methods to maximize yield, purity, and reproducibility³.
ENABLE Biotech has been an early adopter of these next-generation approaches, integrating closed-system TFF, chromatography, and advanced filtration for clients requiring robust, scalable solutions.
Scalability and Process Integration
Scaling exosome production is not simply a matter of increasing vessel size. Vesicle yield and quality depend directly on cell physiology, shear conditions, nutrient exchange, and bioreactor design³. Stirred-tank, fixed-bed, 3D culture systems, and next-generation bioreactors each offer different advantages depending on the target product profile.
Automation and closed-system processing help reduce operator variability, eliminate the need for viral filtration (which exosomes cannot tolerate), and strengthen batch-to-batch consistency⁸. AI-assisted image analysis, multivariate monitoring, and predictive algorithms are beginning to support real-time quality decision-making⁸.
Supplemental technologies—including human platelet lysate, microfluidic culture platforms, and controlled electrical stimulation (“cellular nanoporation”) – show promise but must be integrated carefully into manufacturing workflows to ensure compatibility with clinical-grade processes³.
A CDMO with ATMP experience, such as ENABLE Biotech, can help sponsors navigate this integration, balance innovation with regulatory expectations, and design processes that scale cost-effectively.
Regulatory Considerations for Exosome Therapies
The path to regulatory approval remains complex because exosomes sit at the intersection of cellular therapies, biologics, and nanoparticle-based drug delivery. Regulators are increasingly clear on expectations, but there is still no approved exosome therapeutic⁹, meaning the first movers must define many of the standards that follow.
Key regulatory challenges include:
1. Classification and CMC definition
Sponsors must work with regulators early to clarify how exosomes will be classified—within ATMP frameworks in Europe or as cell- and tissue-based products in the United States—because classification shapes CMC, comparability, and release requirements⁹.
2. Potency and mechanism of action
Potency assays must reflect intended biological activity and be robust enough to support lot release and stability. Regulators are increasingly emphasizing MoA-relevant functional assays over surrogate metrics alone⁹.
3. Comparator controls
Identifying an appropriate control for clinical trials is notoriously difficult. While liposomes are sometimes used as surrogates, they rarely capture the complexity of exosome cargo and biodistribution. Developers increasingly rely on engineered reference lots or standardized functional benchmarks to support regulatory dialogue¹⁰.
4. Stability and logistics
Storage and transport add additional complexity. Exosomes are sensitive to freeze–thaw stress, and optimal storage conditions can vary by cell source¹⁰. Developers are exploring stabilized formulations, cryoprotectants, and standardized cold-chain protocols to maintain potency throughout distribution.
For sponsors, engaging a CDMO with deep ATMP regulatory experience—such as ENABLE—helps ensure alignment with evolving expectations and reduces the uncertainty inherent in first-in-class therapeutics.
Looking Ahead: Building a Path to Commercial Readiness
Today, only a limited number of CDMOs globally have genuine expertise in GMP-grade exosome manufacturing. Selecting the right partner is one of the most consequential decisions a sponsor will make, especially as programs move toward IND-enabling studies.
To succeed, developers need partners who can integrate:
- Next-generation purification technologies,
- Closed and automated manufacturing systems,
- Validated analytical platforms for vesicle characterization,
- ATMP-aligned regulatory strategy, and
- Scalable CMC that balances innovation with robustness.
ENABLE Biotech is among the few European CDMOs offering GMP exosome manufacturing with integrated process development, novel purification strategies, and regulatory support. Our team combines deep experience in ATMP manufacturing, advanced analytics, and cross-jurisdictional regulatory frameworks.
For innovators advancing exosome therapies, selecting a partner with this level of expertise reduces program risk, accelerates timelines, and increases the likelihood that promising vesicle-based therapeutics can reach patients safely and efficiently.
Sources:
- Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science, 367(6478). https://doi.org/10.1126/science.aau6977
- Gu, Z., Yin, Z., Song, P., Wu, Y., He, Y., Zhu, M., Wu, Z., Zhao, S., Huang, H., Wang, H., Tong, C., & Qi, Z. (2022). Safety and biodistribution of exosomes derived from human induced pluripotent stem cells. Frontiers in Bioengineering and Biotechnology, 10. https://doi.org/10.3389/fbioe.2022.949724
- Chen, H., & Li, Q. (2025). Recent advances in scalable exosome production: Challenges and innovations. Chinese Journal of Plastic and Reconstructive Surgery. https://doi.org/10.1016/j.cjprs.2025.05.001
- Busatto, S., Vilanilam, G., Ticer, T., Lin, W.-L., Dickson, D. W., Shapiro, S., Bergese, P., & Wolfram, J. (2018a). Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells, 7(12), 273. https://doi.org/10.3390/cells7120273
- Börger, V., Dittrich, R., Staubach, S., Zumegen, S., Horn, P., & Giebel, B. (2019a). Tangential flow filtration, a potential method for the scaled preparation of extracellular vesicles. Cytotherapy, 21(5). https://doi.org/10.1016/j.jcyt.2019.03.431
- Sidhom, K., Obi, P. O., & Saleem, A. (2020). A review of exosomal isolation methods: Is size exclusion chromatography the best option? International Journal of Molecular Sciences, 21(18), 6466. https://doi.org/10.3390/ijms21186466
- Fernandes, R. P., Ruiz, A. B., Bezemer, S., Detmers, F., Hermans, P., & Peixoto, C. (2025). Targeted isolation of extracellular vesicles from cell culture supernatant using immuno-affinity chromatography. Separation and Purification Technology, 358, 130312. https://doi.org/10.1016/j.seppur.2024.130312
- Whitford, W., & Guterstam, P. (2019). Exosome Manufacturing Status. Future Medicinal Chemistry, 11(10), 1225–1236. https://doi.org/10.4155/fmc-2018-0417
- Wang, C., Tsai, T., & Lee, C. (2024). Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clinical and Translational Science, 17(8). https://doi.org/10.1111/cts.13904
- Tzng, E., Bayardo, N., & Yang, P. (2023). Current challenges surrounding exosome treatments. Extracellular Vesicle, 2, 100023. https://doi.org/10.1016/j.vesic.2023.100023